On May 1 2020, a Facebook user shared the following post, which contained the claim that antiviral medication remdesivir had replaced hydroxychloroquine as a favored hopeful treatment for the coronavirus that causes COVID-19 because the former medication was less expensive than the latter:
As of May 7 2020, the post had been shared nearly 100,000 times — in a Facebook-generated format known to exacerbate the spread of disinformation. As with many memes in this format, contained no citations or other corroborating information, and paired large bold text with a colorful background. (The name of remdesivir is misspelled as “remdesvir” in that post.)
“Hydroxychloroquine is 60¢ a Pill”
Chloroquine or hydroxychloroquine emerged early on in the COVID-19 pandemic as a possible cure for COVID-19. On March 19 2020, the Washington Post explained of interest in and research into chloroquine as a possible COVID-19 treatment:
The [Food and Drug Administration] is considering an expanded trial to see whether chloroquine, a cheap, decades-old treatment for mosquito-borne disease, is effective for treating covid-19.
Chloroquine and hydroxychloroquine had two things going for it above all else — they were invented decades before the pandemic, and are typically very inexpensive. An April 10 2020 StatNews.com article on the cost of chloroquine and hydroxychloroquine provided the respective costs for those medications in a 14-day course (not per pill) at $0.30 and $1 respectively.
Those rock-bottom prices weren’t entirely static, though still relatively low. In the above-linked Washington Post article as of March 19 2020:
Chloroquine is an inexpensive generic drug that has been used for 70 years against malaria and has shown promise in laboratory tests against the novel coronavirus.
It is attracting great interest as a potential treatment and is being studied in China, the United States and Europe. Bayer, which said it discovered the drug in 1934, announced it is donating 3 million doses to the U.S. government. Although about a dozen generic and brand manufacturers have different versions of the drug approved in the United States, according to the FDA website, Bayer is not among them. The company says it is seeking an emergency authorization from the agency so the drug can be given to U.S. patients.
The drug costs as little as 30 cents a pill at retail Canadian pharmacies, according to the website PharmacyChecker.com. In the United States, where drug prices typically are the highest in the world, the retail price is $6.63 per tablet, according to the website Drugs.com.
Based on that snapshot in time, chloroquine or hydroxychloroquine ranged from $0.30 to $6.63 per tablet — still far less expensive than “$1000 a dose.” But it should also be noted that on March 19 2020, StatNews.com reported fluctuations in chloroquine pricing which were seemingly unrelated to the COVID-19 pandemic:
Rising Pharmaceuticals, a New Jersey-based drug company, hiked the price of its chloroquine phosphate tablets 98% between December 2019 and January 2020, according to data provided to STAT by the publishing and analytics company Elsevier, from roughly $3.87 to $7.66 for a 250-milligram tablet.
The price hikes, however, came months before the coronavirus outbreak morphed into a global pandemic, and well before physicians and scientists came to believe chloroquine might prove an effective treatment. In the past two weeks, Rising Pharmaceuticals slashed the price in half as interest in the drug — normally used as an antimalarial — erupted.
Remdesivir ‘Costs $1000 a Dose’?
As for remdesivir’s cost, it was difficult to nail down a specific price tag for that particular medication due in part to variations in how it is administered and why. It’s not even really clear when remdesivir was specifically developed; a COVID-19 specific document [PDF] from maker Gilead indicates it was developed “as early as 2009.”
When we restricted our remdesivir searches back to mid-2019, only three pages of results were returned on Google — suggesting remdesivir was not a widely-discussed medication before COVID-19 gave Gilead its big break. Gilead’s linked PDF suggested remdesivir’s purpose remained under review until recently, and there was no real available fixed price listed anywhere; most mentions of the drug by name involved published research on different applications for it, such as Ebola.
However, we did find a possible source for the claim Remdesivir cost $1,000 a dose. A May 6 2020 Reuters item (published after the Facebook post circulated) made what appeared to be a common claim about Gilead Sciences, Inc.:
The drugmaker [Gilead] earned notoriety less than a decade ago, when it introduced a treatment that essentially cured hepatitis C at a price of $1,000 per pill.
Public outrage over the cost of Sovaldi in 2013 – despite that it was a vast improvement over existing equally expensive therapies – ignited a national debate on fair pricing for prescription medicines that the pharmaceutical industry has fought to deflect ever since.
Reuters began by referencing a 2013 controversy over another medication, Sovaldi, which cost $1,000 a pill (or $1,000 a dose.) From there, Reuters speculated about the cost of remdesivir should it prove to be effective in treating COVID-19:
The Institute for Clinical and Economic Review (ICER), which assesses effectiveness of drugs to determine appropriate prices, suggested a maximum price of $4,500 per 10-day treatment course based on the preliminary evidence of how much patients benefited in a clinical trial. Consumer advocacy group Public Citizen on Monday said remdesivir should be priced at $1 per day of treatment, since “that is more than the cost of manufacturing at scale with a reasonable profit to Gilead.”
Some Wall Street investors expect Gilead to come in at $4,000 per patient or higher to make a profit above remdesivir’s development cost, which Gilead estimates at about $1 billion.
It wasn’t clear how many doses per day the Institute for Clinical and Economic Review expected for remdesivir in COVID-19 trials, or even necessarily how it might be administered. The only use of the word “pill” is quoted above in relation to the $1,000 dose of Sovaldi, and the article’s featured image showed a vial of what looked to be powdered remdesivir labeled for “injection” and captioned:
An ampule of Ebola drug Remdesivir is pictured during a news conference at the University Hospital Eppendorf (UKE) in Hamburg, Germany, April 8, 2020, as the spread of cor onavirus disease (COVID-19) continues.
Assuming remdesivir will be used intravenously, the proposed ten-day course at $4,500 as a maximum suggested cost would work out to $450 per day or dose. However, that price was proposed as the top end of pricing by a third-party group unrelated to Gilead; a separate group proposed a $1 a day cost for remdesivir — bringing it in line with the cost of chloroquine or hydroxychloroquine.
In their undated press release, ICER proposed a range as low as $1 per day of therapy and as high as $450:
Models present prices according to two different paradigms: “cost recovery” approach and traditional cost-effectiveness analysis[;]
Pricing estimates based on preliminary data and will be updated regularly as further data are released and clinical use evolves to include earlier treatment; models will also be used to provide pricing estimates for future treatments as they emerge[;]
Preliminary cost recovery pricing for 10-day course of remdesivir estimated at $10; cost-effectiveness pricing at a commonly used threshold for treatments of large patient populations estimated at a ceiling of $4,500. Policymakers and the public will need to debate most appropriate development and pricing paradigms to be used to achieve rapid development and distribution of affordable treatments for a global pandemic[.]
On the same day Reuters ran their report, Politico reported a separate earlier Gilead pricing controversy, and noted no known pricing ballpark had yet been disclosed by any involved parties:
But Gilead, which suffered through a spate of bad publicity in 2015 for charging $84,000 for a hepatitis C drug, isn’t just under fire over the potential price of its coronavirus treatment. It’s under pressure from Wall Street investors to recoup the $1 billion investment in remdesivir, which has been proven to accelerate recovery from the coronavirus. How Gilead navigates financial pressures from investors and political pressures from Washington may very well determine the mass production and availability of one of the most promising coronavirus drugs on the market.
“An unaffordable drug is completely ineffective,” Democratic Reps. Lloyd Doggett (Texas) and Rosa DeLauro (Conn.) wrote to Health and Human Services Secretary Alex Azar last week, raising questions about remdesivir.
It’s not clear exactly how much the drug will cost. For context, one non-profit that evaluates drug costs says it costs about $9.32 to manufacture a 10-day course of remdesivir treatment for one patient. Calculating the cost of development and trials, the Institute for Clinical and Economic Review says Gilead could charge as little as $390 for the drug. But Wall Street analysts are on an entirely different page, suggesting a price between $5,000 to $10,000, leading to billions in profits.
The FDA’s Emergency Use Authorization (EUA) for Remdesivir
Chloroquine was briefly issued an EUA by the FDA in March 2020; on May 1 2020 (the same date the Facebook post was published), the FDA granted an EUA for remdesivir for COVID-19. That EUA indicated remdesivir was to be used intravenously in patients being treated for COVID-19:
[On May 1 2020], the U.S. Food and Drug Administration issued an emergency use authorization for the investigational antiviral drug remdesivir for the treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. While there is limited information known about the safety and effectiveness of using remdesivir to treat people in the hospital with COVID-19, the investigational drug was shown in a clinical trial to shorten the time to recovery in some patients.
“FDA’s emergency authorization of remdesivir, two days after the National Institutes of Health’s clinical trial showed promising results, is a significant step forward in battling COVID-19 and another example of the Trump Administration moving as quickly as possible to use science to save lives,” said HHS Secretary Alex Azar. “NIH, FDA, and scientists across America and around the world have worked tirelessly with patients to get us this new potential treatment for COVID-19. The seamless cooperation between government and private industry under the President’s all-of-America approach to COVID-19 is getting treatment options to patients in record time.”
The emergency use authorization allows for remdesivir to be distributed in the U.S. and administered intravenously by health care providers, as appropriate, to treat suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease. Severe disease is defined as patients with low blood oxygen levels or needing oxygen therapy or more intensive breathing support such as a mechanical ventilator.
The Meteoric Rise and Fall of Chloroquine/Hydroxychloroquine During the COVID-19 Pandemic
On March 30 2020, we addressed both the sudden and emergent introduction of chloroquine, and its abrupt fall from medical favor:
Chloroquine and hydroxychloroquine received a FDA EUA in mid-March 2020, authorizations withdrawn by late March 2020. In the intervening days, research indicated the drug demonstrated little to no benefit and posed significant risk of life-threatening side effects.
On April 12 2020, the New York Times reported on the cessation of a small study in Brazil involving chloroquine and COVID-19:
A small study in Brazil was halted early for safety reasons after coronavirus patients taking a higher dose of chloroquine developed irregular heart rates that increased their risk of a potentially fatal heart arrhythmia.
[…]
The Brazilian study involved 81 hospitalized patients in the city of Manaus and was sponsored by the Brazilian state of Amazonas. It was posted [on April 11 2020] at medRxiv, an online server for medical articles, before undergoing peer review by other researchers. Because Brazil’s national guidelines recommend the use of chloroquine in coronavirus patients, the researchers said including a placebo in their trial — considered the best way to evaluate a drug — was an “impossibility.”
Ultimately, hopes the inexpensive and decades-old anti-malarials chloroquine and hydroxychloroquine could treat COVID-19 were dashed by immediate observation of “potentially fatal heart arrhythmia” in patients.
That wasn’t news, by the way. Genetic cardiologist Michael Ackerman recounted his reaction to the March 2020 news about trialing chloroquine in a piece called “Antimalarials widely used against COVID-19 heighten risk of cardiac arrest. How can doctors minimize the danger?”:
“I was kind of going crazy in the car,” Ackerman remembers [of listening to a White House press briefing in March 2020]. “My wife was like, ‘Settle down, settle down.’” At the Mayo Clinic, Ackerman treats patients predisposed to heart arrhythmias because of genetic conditions. Chloroquine and hydroxychloroquine, he knows, have a potentially fatal side effect: They can cause a type of irregular heart rhythm that sometimes leads to cardiac arrest. “The side effect is rare—that’s the great news,” Ackerman says. But doctors can’t say just how risky these drugs are for gravely ill COVID-19 patients based on data from other groups of people who have taken them over the decades. The expert on the radio was comparing, “not apple to oranges, but apples to watermelons,” he says.
TL;DR
A Facebook post claimed that remdesivir overtook hydroxychloroquine and chloroquine because the latter therapy cost $0.60 a pill and the former was $1,000 a dose:
Hydroxychloroquine 60¢ a pill, Remdesvir $1000 a dose. Now do you see why they are pushing the New Drug and not the old one?
However, remdesivir was very new and not yet firmly priced at $1,000, a figure which seemed to originate from a previous pricing controversy involving Gilead Sciences, Inc. Hydroxychloroquine and chloroquine costs as little as $0.30, but its experimental use was abruptly halted due to major risks observed in COVID-19 patients. One proposed remdesivir pricing model suggested a cost of $1 a day for the drug should it prove effective, with a high-end proposed at $450 a day (not $1000 a dose).
Ultimately, the post’s base claim — that hydroxychloroquine fell from favor because of remdesivir’s relative cost — was flawed on several levels. Chloroquine and hydroxychloroquine trials ended because of potentially fatal complications, and remdesivir had no set price as it was trialed in COVID-19 patients.
Update, June 29 2020
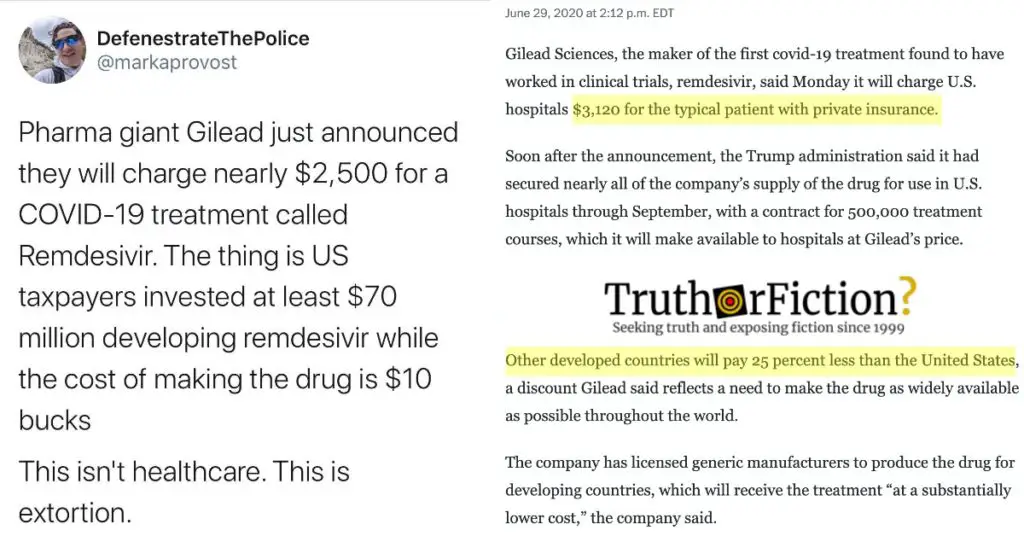
On June 29 2020, Gilead Sciences, Inc. disclosed the anticipated cost of remdesivir: $3,120.
The Washington Post reported:
Gilead Sciences, the maker of the first covid-19 treatment found to have worked in clinical trials, remdesivir, said [on June 29 2020 that] it will charge U.S. hospitals $3,120 for the typical patient with private insurance.
Soon after the announcement, the Trump administration said it had secured nearly all of the company’s supply of the drug for use in U.S. hospitals through September [2020], with a contract for 500,000 treatment courses, which it will make available to hospitals at Gilead’s price.
Other developed countries will pay 25 percent less than the United States, a discount Gilead said reflects a need to make the drug as widely available as possible throughout the world.
The Post also included some objections to remdesivir’s $3,12o price tag (25 percent lower outside the United States):
Rep. Lloyd Doggett (D-Tex.) said Gilead had set “an outrageous price for a very modest drug, which taxpayer funding saved from a scrapheap of failures.” He criticized the Trump administration for not demanding a lower price in its contract for the first 500,000 courses of treatment.
“In a grotesque display of hubris and disregard for the public, Gilead has priced at several thousand dollars a drug that should be in the public domain, said Peter Maybarduk, access to medicines director for the nonprofit group Public Citizen. “Gilead did not make remdesivir alone. Public funding was indispensable at each stage, and government scientists led the early drug discovery team. Allowing Gilead to set the terms during a pandemic represents a colossal failure of leadership by the Trump administration.”
Although the original meme placed the cost at $1000 a dose, the actual cost per dose was just over half of that. Six doses comprised a course of remdesivir for COVID-19, for a total of $3,120 in the United States. Outside the US, the cost was $390 a dose, or $2,340 for the six dose course.
- The COVID-19 Chloroquine Controversy, Explained
- Researcher Behind Retracted Paper Launches Anti-Fauci Campaign
- Would-be coronavirus drugs are cheap to make
- A drug maker recently doubled the price of chloroquine — but in response to the coronavirus pandemic, it’s cutting it in half
- DEVELOPMENT OF REMDESIVIR
- Remdesivir price
- Remdesivir
- Remdesivir
- Will Gilead price its coronavirus drug for public good or company profit?
- Remdesivir helps coronavirus patients — but at what cost?
- Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment
- Small Chloroquine Study Halted Over Risk of Fatal Heart Complications
- Antimalarials widely used against COVID-19 heighten risk of cardiac arrest. How can doctors minimize the danger?
- Gilead sets price of coronavirus drug remdesivir at $3,120 as Trump administration secures supply for 500,000 patients