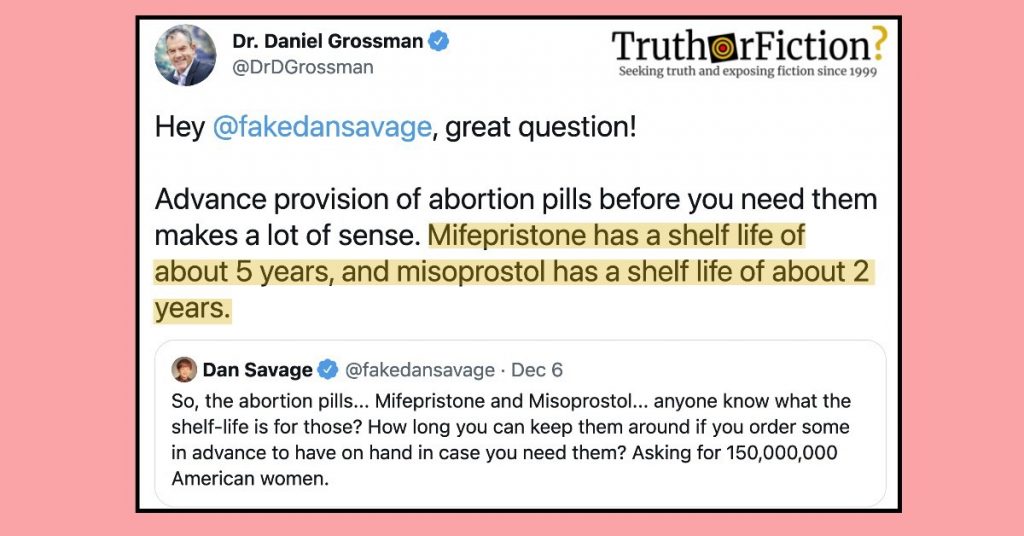
As discourse about the future legality of abortion continued on December 6 2021, an Imgur user shared a screenshot of two tweets addressing the shelf lives of mifepristone and misoprostol — two drugs known to terminate a pregnancy:

In the depicted exchange, advice columnist Dan Savage broadly asked if anyone knew the shelf life of mifepristone and mifeprostol. Public health expert Dr. Dan Grossman responded, indicating that the medications had shelf lives of five and two years, respectively:
Fact Check
Claim: "Mifepristone has a shelf life of about 5 years, and misoprostol has a shelf life of about 2 years."
Description: In a twitter exchange, Public health expert Dr. Dan Grossman indicated that the shelf lives of mifepristone and misoprostol were approximately five years and two years respectively. However, the analysis of the claim pointed out that the storage conditions such as temperature and exposure to moisture could reduce the efficacy of either medication.
Although the tweet above did not receive significant engagement, the Imgur screenshot was viewed more than 100,000 times. A very similar post appeared on Reddit’s r/abortion in May 2019:
What are Mifepristone and Misoprostol?
According to a Food and Drug Administration (FDA) fact sheet for Mifeprex (mifepristone):
Mifeprex is used, together with another medication called misoprostol, to end an early pregnancy. The FDA first approved Mifeprex in 2000. In 2016, the agency approved a supplemental application for Mifeprex based on data and information submitted by the drug manufacturer. After reviewing the supplemental application, the agency determined that Mifeprex is safe and effective when used to terminate a pregnancy in accordance with the revised labeling.
[…]
Mifeprex is approved, in a regimen with misoprostol, to end a pregnancy through 70 days gestation (70 days or less since the first day of a woman’s last menstrual period).
Of misoprostol (a medication also used to prevent ulcers caused by NSAID pain relievers), WebMD said:
This medication [misoprostol] is used to prevent stomach ulcers while you take NSAIDs (e.g., aspirin, ibuprofen, naproxen), especially if you are at risk for developing ulcers or have a history of ulcers. Misoprostol helps to decrease your risk of serious ulcer complications such as bleeding. This medication protects your stomach lining by lowering the amount of acid that comes in contact with it. This medication is also used in combination with another drug (mifepristone) to end a pregnancy (abortion).
An April 2021 paper in the American Academy of Family Physicians’ American Family Physician explained the manner in which the medications were dispensed in concert, and the timeframe during which they could be used to terminate a pregnancy:
Medication regimens using mifepristone and misoprostol are safe and effective for outpatient treatment of early pregnancy loss for up to 84 days’ gestation and for medication abortion up to 77 days’ gestation. Gestational age is determined using ultrasonography or menstrual history. Ultrasonography is needed when gestational dating cannot be confirmed using clinical data alone or when there are risk factors for ectopic pregnancy. The most effective regimens for medication management of early pregnancy loss and medication abortion include 200 mg of oral mifepristone (a progesterone receptor antagonist) followed by 800 mcg of misoprostol (a prostaglandin E¹ analogue) administered buccally or vaginally. Cramping and bleeding are expected effects of the medications, with bleeding lasting an average of nine to 16 days. The adverse effects of misoprostol (e.g., low-grade fever, gastrointestinal symptoms) can be managed with nonsteroidal anti-inflammatory drugs or antiemetics. Ongoing pregnancy, infection, hemorrhage, undiagnosed ectopic pregnancy, and the need for unplanned uterine aspiration are rare complications. Clinical history, combined with serial quantitative beta human chorionic gonadotropin levels, urine pregnancy testing, or ultrasonography, is used to establish complete passage of the pregnancy tissue.
Medication management of early pregnancy loss and medication abortion has become increasingly common since the U.S. Food and Drug Administration (FDA) approval of mifepristone (Mifeprex) in 2000. Medication abortion now accounts for 60% of all abortions completed before 10 weeks’ gestation.
In sum, mifepristone and misoprostol are oral medications, one of which is also used to prevent stomach ulcers (misoprostol). Both medications are considered safe and effective for outpatient use in terminating a pregnancy up through “77 days’ gestation.”
What’s a Shelf Life in Relation to Medication, and How Does it Relate to Mifepristone or Misoprostol?
Drugs.com’s entry on “expired” medications provided context about shelf lives overall:
What does an expiration date mean?
The expiration date is the final day that the manufacturer guarantees the full potency and safety of a medication. Drug expiration dates exist on most medication labels, including prescription, over-the-counter (OTC) and dietary (herbal) supplements. U.S. pharmaceutical manufacturers are required by law to place expiration dates on prescription products prior to marketing.
For legal and liability reasons, manufacturers will not make recommendations about the stability of drugs past the original expiration date. However, for most drugs, it’s just an arbitrary date, usually 1 to 5 years out, that the manufacturer selects to test drug stability. Once the container of medication is opened after production, that expiration date is no longer guaranteed.
A December 2014 paper in the journal PLOS One, “Instability of Misoprostol Tablets Stored Outside the Blister: A Potential Serious Concern for Clinical Outcome in Medical Abortion,” addressed inherent risks of deterioration when its manufacturer’s packaging was damaged:
In such conditions, at the time of drug intake, a decrease in active misoprostol components associated with an increase in inactive degradation products is expected to occur. Correct dosing is of crucial importance for clinical procedures that rely on misoprostol such as medical termination of pregnancy, medical management of miscarriage, and cervical ripening, because dosage recommendations for misoprostol in these indications are based on the lowest effective dose, with the intention to keep side effects at a minimum. Consequently, treatment guidelines do not contain a ‘safety margin’ to compensate for a reduced content of the active ingredient due to degradation.
[…]
Exposure of Cytotec tablets to ‘typical’ European levels of air and humidity results in significant time-dependent changes in physical and biological composition that could impact adversely upon clinical efficacy. Health professionals should be made aware of the degradation of misoprostol with inappropriate storage of misoprostol tablets.
Shelf Life of Misoprostol
Grossman’s response to Savage’s tweet indicated the shelf life of misoprostol was “about [two] years.”
“Two years” was the precise figure cited in a United States Agency for International Development (USAID) document [PDF] pertaining to misoprostol. The Reproductive Health Supplies Coalition [PDF] referenced a slightly broader window, from 18 months to 36 months (or three years):
Misoprostol is available in tablet form, and marketed products typically have a shelf life of 18 to 36 months when stored below 25°C to 30°C (77°F to 86°F) in a dry area.
Misoprostol directives often note that storage conditions and temperature affect the safety and efficacy of stored tablets.
Shelf Life of Mifeprestone
Grossman added that the shelf life of mifeprestone was “about [five] years.”
A document on reproductiveaccess.org, “Mifepristone Ordering Information,” [PDF] read:
Shelf life of mifepristone is 18 months.
However, an Indian pharmaceutical resource referenced a five year shelf life, as Dr. Grossman did in the tweet. Medicines.org.uk listed the shelf life of mifeprestone as four years; a separate UK source [PDF] indicated a shelf life of three years.
A 2016 World Health Organization (WHO) study [PDF] from 2016 discussed variables in the shelf life of mifeprestone, reporting:
Misoprostol is a viscous oil, extremely susceptible to degradation. This is ameliorated by using a 1% dispersion of misoprostol in hydroxypropyl methyl cellulose (HPMC), which is considerably more stable and allows the manufacture of tablets with a shelf life of several years at room temperature. Nevertheless, exposure to water has been shown to be the principal driver in the degradation of misoprostol in tablets, and can occur during manufacture through the use of inappropriate excipients or inadequately controlled environmental conditions. Tablets can also be exposed to moisture depending on their packaging. Polyvinyl chloride (PVC) or polyvinylidene chloride (PVDC)/aluminium blister packs do not provide adequate protection against penetration by moisture, a double aluminium blister pack is therefore recommended.
Estimates for mifeprestone’s shelf life varied quite a bit, with some tablets being rated as safe for five years. Storage conditions for mifeprestone (like misoprostol) were contextually important, with exposure to moisture cited by the WHO as an accelerating factor in reducing efficacy.
Summary
A Twitter exchange between Savage and Grossman about the shelf life of mifeprestone and misoprostol was shared to Imgur in December 2021, against the backdrop of concerns about reduced access to abortion. Grossman’s response — hampered by Twitter’s character limits — indicated that misoprostol had a shelf life of approximately two years, and mifeprestone could be safely stored for up to five years. As approximations, Grossman’s tweet was accurate, and the information useful for individuals who sought to store the medications in question for potential future use. We rated the claim Decontextualized, not because of any inaccuracy in the tweet, but to highlight controllable variables such as temperature and exposure to moisture that could reduce the efficacy of either medication when used together.
Update, April 24 2023, 4:59 PM:
On April 21 2023, the United States Supreme Court “granted emergency requests” from the Biden administration and a drug manufacturer, temporarily staying the impact of a lower court judge’s attempt to rescind FDA approval of mifepristone:
The Supreme Court on [April 21 2023] preserved women’s access to a drug used in the most common method of abortion, rejecting lower-court restrictions while a lawsuit continues.
The justices granted emergency requests from the Biden administration and New York-based Danco Laboratories, maker of the drug mifepristone. They are appealing a lower court ruling that would roll back Food and Drug Administration approval of mifepristone.
On March 14 2023, we published a fact check addressing claims United States District Judge Matthew Kacsmaryk “tried to hide” a scheduled hearing pertaining to FDA approval of mifepristone. That analysis described the initial lawsuit leading to the “lower court ruling,” and explained:
Above the Law identified “the Northern District of Texas’s Amarillo section, where the GOP parked conservative activist Matthew Kacsmaryk” as “the premiere destination for judge-shopping.” It described the crux of the hearing in question — a November 2022 lawsuit filed to overturn the Food and Drug Administration (FDA)’s authorization of the use of “abortion pills” like mifepristone — and described Kacsmaryk’s suppression efforts as “inappropriate”[.]
On April 7 2023, the Biden administration issued a press release about the initial ruling in Texas. In its entirety, it read:
[On April 7 2023] a single federal district judge in Texas ruled that a prescription medication that has been available for more than 22 years, approved by the FDA and used safely and effectively by millions of women here and around the world, should no longer be approved in the United States. The Court in this case has substituted its judgment for FDA, the expert agency that approves drugs. If this ruling were to stand, then there will be virtually no prescription, approved by the FDA, that would be safe from these kinds of political, ideological attacks.
The prescription medication in question in this case is used for medication abortion, and medication abortion accounts for over half the abortions in America. The lawsuit, and this ruling, is another unprecedented step in taking away basic freedoms from women and putting their health at risk. This does not just affect women in Texas – if it stands, it would prevent women in every state from accessing the medication, regardless of whether abortion is legal in a state. It is the next big step toward the national ban on abortion that Republican elected officials have vowed to make law in America.
My Administration will fight this ruling. The Department of Justice has already filed an appeal and will seek an immediate stay of the decision. But let’s be clear – the only way to stop those who are committed to taking away women’s rights and freedoms in every state is to elect a Congress who will pass a law restoring Roe versus Wade. Vice President Harris and I will continue to lead the fight to protect a woman’s right to an abortion, and to make her own decisions about her own health. That is our commitment.
On April 10 2023, NPR described how “a pair of contradictory decisions by federal judges” left mifepristone in somewhat of a legal gray area, noting that mifepristone was used in “half of all abortions,” and to “manage miscarriages”:
Access to a common abortion medication currently hangs in the balance in a pair of contradictory decisions by federal judges, setting the stage for the most significant legal action on abortion since the overturning of Roe v. Wade [in 2022].
Mifepristone is widely used across the U.S. to end pregnancy in the first 10 weeks of gestation. About half of all abortions nationwide are performed using mifepristone as the first of a two-pill regimen. The drug is also commonly used to help manage miscarriages.
The name-brand drug Mifeprex was first approved by the Food and Drug Administration more than 20 years ago. Since then, it has been used millions of times, and major medical groups say it has a strong safety record. A generic version was approved in 2019.
On April 21 2023, British newspaper The Guardian addressed “what [was expected to come] next,” with respect to judicial wrangling over the legality of mifepristone:
The supreme court has fully stayed Kacsmaryk’s order [in their April 21 2023 ruling]. The case now returns to the fifth circuit, which has scheduled oral arguments for 17 May [2023], and [the matter] will very likely make its way back to the supreme court … Even a supreme court ruling should have little impact in states where abortion is already banned: neither misoprostol nor mifepristone has been legally available for abortions in these states since bans came into place after the fall of Roe.
While misoprostol – which helps to empty the uterus by causing the cervix to soften and dilate, and the uterus to contract – can be used safely on its own, it is less effective. Misoprostol-only abortions result in successful termination 88% of the time, and with more complicated side-effects and more need for follow-up care, recent research has shown.
Some of the main abortion providers – including Planned Parenthood, Carafem and Abortion Delivered – have confirmed that they are prepared to prescribe a misoprostol-only regimen for abortions in the event of mifepristone being pulled off the market. Aid Access, the international group that ships abortion pills to the US, trialled misoprostol-only regimens for abortions during the pandemic, with success.
Update, September 8 2023, 3:45 PM:
On September 8 2023, a mifepristone distributor (Danco Laboratories) filed a petition requesting that the Supreme Court overturn a lower court’s ruling on the medication.
CNN’s coverage of the filing indicated that mifepristone remained “available and not subject to restrictions the lower courts have said should be imposed on its use until all legal challenges are resolved” of the April 2023 order. The network further reported that the earliest anticipated activity on the filing was likely to take place in mid-2024:
… US District Court Judge Matthew Kacsmaryk, a Trump appointee, issued a broad ruling blocking approval of the drug as well as the changes the FDA made in subsequent years to make the drug more accessible. In a preliminary ruling, the federal appeals court scaled back on the district court ruling but said the drug could be restricted while the appeal process played out. At that juncture in the case, supporters of the drug asked the Supreme Court to freeze the order while the appeals process played out.
The justices agreed to do so, protecting access to the drug as the case went back to the appeals court.
After [an August 2023] ruling, the Biden administration and the manufacturer had several weeks to file their appeal under normal procedures, but chose to file early perhaps to ensure that the justices had the time to add the issue to their calendar this term, meaning a likely ruling in 2024.
CNBC’s reporting explained that if the Supreme Court agreed to “take the case,” mifepristone would stay on the market pending an anticipated ruling. If the Court refused it, restrictions imposed by a lower court would go into effect:
Should the Supreme Court take the case and uphold the appeals court decision, mifepristone will remain on the market in the U.S. but patients will face more barriers to accessing the medication.
If the high court declines to take the case, the appeals court restrictions will go into effect.
A September 8 2023 Courthouse News Service report on future Supreme Court cases speculated that “the steep consequences of the lower court rulings make it more likely the justices will agree to hear the case,” stipulating that there were “no guarantees” in that regard.
- Shelf Life of Mifepristone and Misoprostol | Imgur
- Shelf Life of Mifepristone and Misoprostol | Twitter
- Why the Supreme Court may look to China as it reconsiders Roe v. Wade
- Shelf-Life of Mifepristone & Misoprostol? | Reddit
- Mifeprex (mifepristone) Information
- Misoprostol - Uses, Side Effects, and More
- Mifepristone and Misoprostol for Early Pregnancy Loss and Medication Abortion
- Drug Expiration Dates - Are Expired Drugs Still Safe to Take?
- Instability of Misoprostol Tablets Stored Outside the Blister: A Potential Serious Concern for Clinical Outcome in Medical Abortion
- Misoprostol | USAID
- Mifepristone Ordering Information
- Misoprostol for maternal health
- Mifepristone Shelf Life
- Mifepristone Shelf Life
- Mifepristone Shelf Life
- Quality of misoprostol products
- Supreme Court preserves access to abortion pill for now
- Texas Judge Sought to ‘Hide’ Abortion Pill Hearing Information
- What's next for the abortion pill mifepristone?
- Statement from President Joe Biden on Decision in Alliance for Hippocratic Medicine v. FDA
- Mifepristone maker asks Supreme Court to make ultimate decision on abortion drug in 2024
- Abortion pill company asks Supreme Court to decide mifepristone case
- Slim Supreme Court docket could see headliners on abortion and social media in coming months